Abstract
Background
Adult T-cell leukemia-lymphoma (ATL) is an aggressive mature T-cell neoplasm caused by human T -cell leukemia virus type 1 (HTLV-1). Up to 5% of infected individuals develop to ATL. HTLV-1 preferentially infects CD4 + T cells, and stimulates cell proliferation and prevents cell death by apoptosis. The viral oncogene-encoded proteins, Tax and HBZ, play important roles in viral infection and cell immortalization. However, the host factor of developing from carrier to patient is not clear.
Results
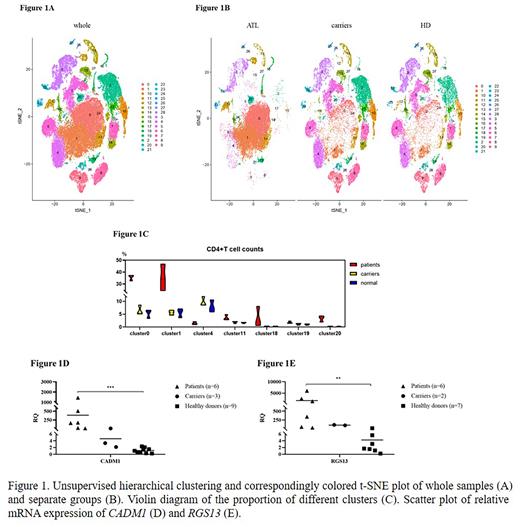
To characterize the heterogeneity of ATL patients, we performed single-cell RNA-sequencing (10x Genomics) analysis on single cell suspensions isolated from PBMCs of nine samples, including three ATL patients, three HTLV-1 asymptomatic carriers as well as three healthy donors (HD). We acquired 82977 high-quality cells with a median of 1718 genes detected per cell. Unsupervised clustering using Seurat followed by visualization in t-Stochastic Neighbor Embedding (t-SNE) identified 29 distinct cell clusters (Figure 1A). The single cell profiling of ATL patients were significantly different from that of carriers and healthy donors, while the latter two had little difference (Figure 1B). Based on singleR packages and marker genes of each cluster, 4 major cell populations (T cells, NK cells, B cells and myeloid cells) and other rare cell types were annotated, such as erythrocyte cluster and eosinophils cluster.
We observed an enrichment of CD4 + T cell from patients in 4 cluster (Figure 1C), which proportion of cells was higher than that of carriers and healthy donors. According to cell type annotation, cells from cluster 11 were CD4 + CD25 + Foxp3 + Treg cells. Based on the increasing proportion of cluster 11 in healthy people, carriers and patients, without significant statistical differences, we assumed that Foxp3 + Treg cells were involved in the evolution of ATL tumor cells. That was identical with published literatures.
To investigate the differences between tumor and normal CD4 + T cell, the gene expression was compared among 7 clusters of CD4 + T cell from three groups. Using a threshold of p value < 0.05 and | fold change| >2. Through integrated analysis, we identified 26 commonly upregulated genes (gene expression level: patients > carriers > HD) and 9 downregulated genes (gene expression level: patients < carriers < HD. To further analyze the biological function of the common DEGs, gene ontology (GO) analysis showed that these genes could be mainly categorized into plasma membrane and protein binding.
Subsequently, we validated the mRNA expression level of upregulated common DEGs among three groups by qRT-PCR. The isolated CD4 + T cell using CD4 microbeads of a total of 6 patients, 3 carriers and 9 normal specimens were included. The result showed that the mRNA expression levels of gene CADM1 and RGS13 in patients were higher than those in carriers and healthy donors, although there was no statistical difference between patients and carriers, and the expression levels of carriers tended to be higher than those in normal people (Figure 1D and E). Previously, CADM1 has been revealed to be highly expressed in HTLV‐1‐infected CD4 + T cells. Our study confirmed this result by single-cell profiling. RGS13, a member of the regulators of G protein signaling (RGS) family, participates in cellular communication. The role of RGS13 in ATL needs to be investigated.
Conclusions
This study is the first time to analyze the single-cell RNA level of ATL patients, HTLV-1 virus carriers and normal people. The peripheral blood cell composition of the patient is significantly different from that of the carriers and healthy donors, while it is similar between carriers and normal people. CD4 + T cells are the main cell population of patients. The proportion of CD4 + CD25 + Foxp3 + Treg cells increased gradually in healthy people, carriers and patients. DEGs analysis showed that CADM1 and RGS13 were highly expressed in CD4 + T cells of patients, followed by carriers, validated by 18 clinical samples. However, the molecular mechanism of RGS13 in the process from HTLV-1 infection to ATL needs to be further studied.
Hu: Astellas Pharma, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal